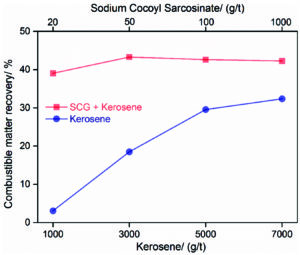
Combustible matter recovery using coconut oil-based amino acid surfactant Sodium Cocoyl Glycinate and kerosene as a flotation collector verses using only kerosene. (Image: Niu, et al.)
Three crucial studies experimented with chemicals and pH, and found possible ways to improve recovery, meet some sustainability goals, and cut costs
by jesse morton, technical writer
Three critical coal flotation studies from 2021 show that the scientific establishment in countries that still prioritize coal power is finding ways to safely improve recovery.
Of the three studies, one concluded a coconut oil-based surfactant could be used with traditional collectors to increase recovery. A second concluded a compound historically used as a preservative in cosmetics outperformed traditional collectors. Both the experimental surfactant and collector are relatively safe and environmentally friendly, and their use in flotation might help operations attain some ES&G goals.
Two of the three studies found lower pH gives better recovery. One found a pH of 4 and the other found a pH of roughly 3 to deliver the best results. The latter found pH determines both the float rate and recovery rate. It concluded pH determines the so-called isoelectric point, which is when particles and bubbles join.
With prices for hydrocarbons rising, all three studies contain information that could possibly help an operation save money and recover more coal.
Coconut oil-based Surfactant Improves Recovery
A study1 out of China demonstrated that Sodium Cocoyl Glycinate (SCG), an amino acid surfactant, when used with kerosene, as a flotation collector, and sec-octyl alcohols, as a frother, produced a significantly higher yield of clean coal than flotation with only kerosene and frother.
Coconut oil-based SCG, with a molecular formula of R-CO-NH-CH2-COONa, has low biological toxicity, can be degraded by microorganisms, and “is the best performing natural amino acid surfactant,” the study said.
The study involved several lab-scale experiments that used aerated flotation system for processing low-grade coal with the kerosene and alcohols, the control group; and low-grade coal with SCG, kerosene, and the alcohols, the experimental group.
For the control group, “the dosages of kerosene were 1,000, 3,000, 5,000 and 7,000 (grams [g] per metric ton [mt]), and meanwhile sec-octyl alcohols usage was 500 g/mt through all the tests,” the study said. For the experimental, “the dosages of collector and frother were fixed at 5,000 g/mt and 500 g/t with SCG concentrations of 20, 50, 100 and 1,000 g/mt.”
The flotation cell was aerated at 0.35 m3/h. “The speed of impeller was 1,900 rpm and the pulp density was 60 g/liter.”
First, some baselines were established by running the process on gangue only, and then on raw coal only.
For the control group, roughly 90 g of raw, low-grade coal was pre-wetted in the cell for a minute. Kerosene was then injected. Two minutes later, the alcohol frother was added. Thirty seconds later, the aeration was activated. “The concentrate and tailings were collected, dried and weighted,” the study said.
For the SCG group, “there was an extra step of SCG addition between the pre-treatment and kerosene injection and this process lasted for 1 min.”
The metrics obtained for comparing the two groups were bubble attachment time, hydrophobicity estimates, surface tension effect estimates, emulsification effect estimates, froth layer size, and bubble size and distribution.
To measure bubble attachment time, an Induction 2015E tester designed by the University of Alberta was used.
The experimental group had a half-of-success adhesion time of 100 ms. The control group had a time of 270 ms. (Raw coal alone had a time of 613 ms.) “The presence of SCG cut down the attachment time largely by making coal particle surfaces more hydrophobic,” the study said.
Infrared spectroscopy found “both hydrophobic and hydrophilic functional groups were found in the molecular structure of SCG.” When SCG is mixed with kerosene, however, the result is increased hydrophobicity.
“Hydrophilic functional groups of SCG adsorbed with the hydrophilic sites on the coal surface through hydrogen bonds, leaving the hydrophobic ends exposed to the bubbles,” the study said. “This made a great difference at the content of hydrophobic functional groups on the coal surface, and shorten the attachment time, which enhanced the hydrophobicity of the coal surface.”
A surface tensiometer was used to measure surface tension for the control and SCG groups. A 19.69-mm platinum ring “was vertically inserted into the solution and then lifted to determine the air-liquid interface tension.”
The results suggested surfactant SCG adsorbed at the gas-liquid interface, decreasing the surface tension. “SCG could reduce the surface tension of the solution efficiently, which might be beneficial to bubbles generation when a large dosage of oily collectors is applied,” the study said. “Moreover, the reduction of the surface tension may have an effect on the emulsification of the collector.”
To determine the effects of SCG on emulsion of kerosene and water, turbidity was measured in the control and experimental groups using a ZDK turbidimeter. “Droplet size distribution was determined using an Occhio nano 500 XY.”
The results showed the “surfactant would also work on the oil-water interface. Its polar end would insert into the water phase, and meanwhile the non-polar group enters the oil phase to form a directional absorption,” the study said. “The reduction of interfacial tension between water and oil cuts down the energy required to form the emulsion making the emulsification easier.”
To determine the height of the froth layer, a setup was used consisting of a “30 cm-height column, solution zone, quartz sand core and a float flowmeter.” The setup was aeriated at a fixed flow of 0.35 m3/h.
“For each test, the same amount of water was added into the column,” the study said. “After that, kerosene, sec-octyl alcohols and SCG were injected, and then” the air valve was turned on and the maximum height of froth layer, after 40 s of aeration, was measured.
The results showed “the maximum height of froth layer without SCG was relatively low, at 7 cm, and its half-life was about 2 s,” the study said. “However, the maximum height increased to around 16 cm when SCG was added and its half-life slightly prolonged to around 3 s, conveying that the addition of SCG contributed to the generation and maintenance of bubbles.”
To determine the distribution and the size of the bubbles, “photos of the froth layer during low-rank coal flotation were prepared, and then analytical software, Image-J, was used to extract the shape information from the images.”
The results showed “the maximum and minimum diameters of bubble were 5.3 and 0.4 mm in presence of SCG, as well as 6.7 and 1.25 mm without the surfactant,” the study said.
Roughly “80% bubbles fell into the size range of 0.82 to 2.51 mm for the SCG curve,” and without SCG “the bubbles with diameters of 3.30 to 5.41 mm took up 80%,” it said. The average diameter of bubble was “4.32 mm for SCG-absent group, which was about two times larger than SCG-addition group’s (1.21 mm).”
This means “smaller bubbles dominated after SCG was applied.”
The study concluded “a small amount of SCG (50 g/mt) could improve recovery in low-rank coal flotation, and increase the concentrate yield by about 12% with its ash content roughly unchanged.” There is a point of diminishing returns. “The concentrate yields plateaued at 35% when more SCG was employed, and though a light upswing was found on the corresponding ash contents of concentrate, they were never greater than 10%.”
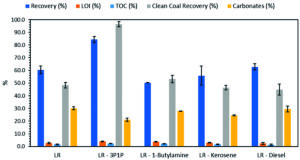
As a collector for low-rank (LR) coal, 3-phenyl-1-propanol (3P1P) outperforms ‘natural flotation,’ butylamine, kerosene, and diesel. (Image: Blanco-Flores, et al.)
Preservative Beats Hydrocarbon Reagents
A study2 out of Mexico found that 3-phenyl-1-propanol outperformed butylamine, kerosene and diesel as a reagent in flotation of a low-rank coal.
“The volume of collector for optimal Recovery was 84.5 µL” per 0.5 g of coal, the study said. “The maximum Clean Coal Recovery was achieved at pH 4; 2.5 min. of flotation time, and 87.9 µL of 3-phenyl-1-propanol.”
In the study, “Recovery” refers to any coal-bearing mineral concentrate, and “Clean Coal” recovery references the more-desirable grades of concentrate.
Described as a non-ionic amphiphile, meaning it can be both hydrophilic and lipophilic, 3-phenyl-1-propanol can be found in nature and has historically been used as a preservative in cosmetics due to its antibiotic properties. As a reagent in coal flotation, it interacts with both hydrophobic and hydrophilic parts of the coal particle.
It improves recovery by, among other things, modifying the zeta potential, or the ionic characteristics, of the coal particle surface, which helps with bubble attachment.
The study looked at floating “a low-rank carbonaceous mineral obtained from the Sabinas basin in Coahuila, Mexico.” The reagents used “were of ACS grade and were obtained from Sigma ldrich.”
The mineral was thoroughly assessed to determine its characteristics.
X-ray diffraction analysis used a 30-kV D8-Advance X-ray diffractometer. It found that “in general, silicates are predominant, whereas pyrite and sphalerite exhibit low concentrations; the predominant phase is quartz (59.2%), followed by calcite (20.4%) and orthoclase (17.9%),” the study said.
The pH was determined by potentiometry, using a Thermo Scientific electrode. It found “the pH value is slightly basic, which is consistent with the presence of carbonates,” the study said.
The loss on ignition (LOI) was determined by the gravimetric method. The mineral was washed, dried and then heated at high temps for set times. “The LOI was determined at 1,100° C for 4 h.”
A Bernand calcimeter determined the carbonate content of samples.
The mineral was treated with chemicals and then subjected to Fourier Transform Infrared Spectroscopy. “A Thermo Scientific Nicolet iS20 FT-IR spectrometer with an ATR and a spectral resolution of 0.4 cm to 1 was used.” The surface groups were studied. The signals obtained “are commonly identified in low-rank or oxidized coal.”
Zeta potential measurements were taken using a Zetasizer Nano ZS90. A separation process was performed beforehand to remove large particles.
The contact angle of 3-phenyl-1-propanol to graphite, as a hydrophobic surface model, was estimated. Water was used as a reference. A Ramé-Hart contact angle instrument was used to compare photographs and measurements. Findings suggested 3-phenyl-1-propanol has “a much lower contact angle, showing a tendency to interact with hydrophobic material.” Yet the angle was “different from zero,” suggesting “the molecule presents a little grade of hydrophilicity.”
The tests to compare the floatability performance of the reagents were performed in a Hallimond tube. A mineral sample was added to the flotation cell and wetted, followed by 100 µL of collector. “The dosage of kerosene and diesel was the same as 3-phenyl-1-propanol,” the study said. “The amine dosage was 10-3 mol/L.”
“The pulp was conditioned for 3 min. at 800 rpm, the cell was then filled with water, and the concentrate was collected during 2 min.; then it was dried and weighed,” the study said. “Experiments were conducted using nitrogen flow rates of 8-20 mL/min. and constant agitation (400 rpm).”
The floatability performance results for the different reagents was compared.
The microflotation tests found that “1-butylamine, kerosene and diesel were not effective for the flotation of coal because the Clean Coal Recovery of each one (53.2%, 46.4% and 44.8%, respectively) is not significantly superior to the value obtained in the natural flotation (48.3%),” the study said.
“However, 3-phenyl-1-propanol allows to recover the 96.4% of the coal in the mineral,” it said.
The molecular structure of 3-phenyl-1-propanol “allows a better interaction with aliphatic carbon chains and aromatic rings on the coal surface,” the study said. “At the same time, the alcoholic hydroxyl group provides hydrophilicity to the molecule, which allows it to interact with the oxidized coal.”
The tests found 3-phenyl-1-propanol modified zeta potential at pH 2, 3, 12 and 13. That “suggests that there is a modification of the mineral surface due to its interaction with the collector.” The suggestion was supported by FT-IR spectra analysis.
Coal flotation parameters were optimized using the Box–Behnken methodology.
It showed “only the flotation time and the dosed volume of collector have a statistically significant effect on Recovery,” the study said. For Clean Coal Recovery, pH and dosed volume had “a statistically significant effect.” Clean Coal Recovery decreased when pH increased.
Thus, while “pH does not exhibit a significant influence on the mineral Recovery,” it “influences significantly the carbon content,” the study said. “The optimal pH for Clean Coal Recovery is an acid value whereas, for the Recovery, it is a basic value.”
The results suggested a point of diminishing returns on float time. “Carbon content in the mineral can be recovered in a certain time, after which no more coal floats and only gangue keeps floating,” the study said. “For this reason, a difference is observed between the optimal times.”
Ultimately, 3-phenyl-1-propanol outperformed the traditional hydrocarbon reagents. “For this type of coals, the use of non-ionic amphiphilic molecules as collectors are more likely to be effective,” the study said.
The low-grade coal originally had negative zeta potential, which was modified by 3-phenyl-1-propanol. General mineral recovery was not significantly affected by pH, but was enhanced by float time and the size of the dose of reagent. Optimal clean coal recovery, however, required a pH of 4, 2.5 minutes flotation, and a slightly bigger dose of 3-phenyl-1-propanol.
One of the study’s authors said the study showed 3-phenyl-1-propanol is a potential collector. “Dosage tests and optimization study in real flotation cells are necessary for practical conclusions, but due to the non-ionic amphiphilic molecular structure and the frother properties, it is likely that good results in this kind will be obtained,” said Dr. Alien Blanco Flores, professor, mechanical engineering, Tecnológico de Estudio Superiores de Tianguistenco.
The reagent is relatively safe compared to others, making it possibly more attractive to miners targeting sustainable development goals, she said. “This reagent is less toxic and dangerous than commonly used hydrophobics such as kerosene or diesel,” Blanco Flores said.
“The common use of this substance is as a preservative in cosmetics, so, it is not toxic under a normal exposition; however, for industrial manipulation, we advise to use mask and gloves,” she said. “It is not expensive in comparison with advanced collector reagents for coal that have not performed better than this alcohol.”
Since the study was published, Blanco Flores has explored the viability of other possible advanced reagents, but said she has yet to find one truly competitive with 3-phenyl-1-propanol.
pH Regulates Float Rate, Recovery
A study3 by researchers from Turkey and Poland found that the flotation and recovery rate of coal can be regulated by changing the pH in the flotation cell. The study said “pH influences the surface electrical potential of the coal particles, measured as zeta potential, and also coal hydrophobicity, measured as contact angle.”
Flotation rate “is a complex function” of, for the most part, zeta potential and the “flotation equilibrium constant.” Those two parameters and the “reverse process rate constant,” or the rate at which particle-bubbles disintegrate, “can be used for comparison of different flotation systems.”
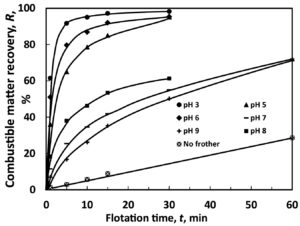
The lower the pH, the greater the recovery, the less float time required. (Image: Ömer Canıeren, et al.)
The study looked at a “naturally hydrophobic coal” from the Zonguldak mine in Turkey. “This coal can be specified as a low-volatile bituminous coal,” the study said. The ash content was 12%.
For the study, numerous sample batches were floated. Each batch, weighing 100 g and made up of “−106 + 38 µm size fraction,” was put in a lab-scale, 1-dm3 Denver flotation cell with tap water and mixed for 5 min. Acid or sodium hydroxide was added to adjust the pH.
An “Aerofroth 65 (AF65) polyglycol-based frother was added and stirred for 1 min.”
Froth products and tailings were filtered, dried, weighed and then sampled for analysis.
The froth products were analyzed for ash content. From each batch, 10 g of dried sample was ground and burned for hours. The resulting ash was weighed to determine the ash content. “The recovery, as combustible matter, was calculated using the weight of the froth product and feed as well as the ash content of the froth product and feed.”
To determine zeta potential, a sample of each batch was ground in a lab ball mill, and screened through a sieve. The undersize was suspended in “both pure water and frother solutions separately.” The coarse particles settled.
“Then, a small amount of sample was taken from the top of the suspension and transferred to the measurement cell,” the study said. A Zetasizer Nano-ZS gave the zeta potential. “Three measurements for each coal suspension were performed and the average value of the measurements was calculated.”
Contact angles for each sample batch “were measured by the sessile drop method using a Contact Angle Meter,” a CAM-100, the study said. “Measurements were carried out at the different pHs,” but at a constant 80-g/Mg dose of frother.
The findings basically showed how pH significantly influenced the other factors and drove the flotation rate and recovery. That suggests that in equations for determining flotation rate and maximum recovery, the other factors can be lesser variables, the study said.
For example, “the investigated coal was naturally hydrophobic with maximum interpolated contact angle equal to about 72° at pH 4,” the study said. “The contact angle was sensitive to pH and varied between 60° and 72° in the presence of applied frother,” it said. “Frother-only flotation of the investigated coal
was pH-dependent.”
When slightly acidic or slightly basic, the float “environment” became “very unstable,” and recoveries had to be entered as averages, the study said.
The findings showed “the frother does not influence much the values of zeta potential.”
Instead, “the zeta potential data, as a function of pH, can be approximated with an empirical equation” that has been previously published, the study said.
“Much more useful is application of the measured and calculated physical parameters for theoretical kinetico-thermodynamic considerations,” it said. And the applicability is broad, it said.
Previous literature proves the kinetics, equilibrium, and maximum recovery rate of both thermodynamic and fractionated flotation processes are “practically identical” so long as “fractionated flotation is not performed exceptionally long.” Thus, the findings apply to both types of flotation, the study said.
The study found that when pH is the driving parameter, “the equilibrium constant of each test is different” and the Gibbs potential, or standard chemical potential, “is not constant.” The Gibbs potential changes “resulting from changes of pH of the flotation environment.”
Further, in the tests, the recoveries were “regulated with pH leading to changes of hydrophobicities in the air/bubble/particle water system,” it said.
Previous literature suggested “the hydrophobicity of a three-phase system is measured as contact angle.” Contrarily, the study found “hydrophobicity change can be characterized with other parameters; for instance: surface potential, which is the more influential factor in flotations regulated with pH.”
The study said that “it is obvious that the standard state of the particle-bubble aggregates occurs … at the pH of the solution when the particle/aqueous solution reaches the so-called isoelectric point,” which for the study was 2.75. Reaching the isoelectric point is dependent on pH.
1 Nui, Chenkai; et al. (2021) Insight into the low-rank coal flotation using amino acid surfactant as a promoter. DOI: https://doi.org/10.1016/j.fuel.2021.121810.
2 Blanco-Flores, Alien, et al. (2021) Coal flotation in a low-rank carbonaceous mineral using 3-phenyl-1-propanol as a collector reagent. DOI: https://doi.org/10.1016/j.fuel.2021.121363.
3 Ömer Canıeren, et al. (2021) Evaluation of kinetics and thermodynamics of a naturally hydrophobic coal flotation in the presence of frother and regulated with pH. DOI: https://doi.org/10.1080/19392699.2021.1964490.

StackCell design features a tank within a tank, giving one-way isolation of fluid between tanks and increasing air bubble concentration. (Image: Eriez)
Compact, Two-stage Flotation Solution Gives Recovery Rate of 75%
By Jesse Morton, Technical Writer
In field testing, a pilot-scale StackCell circuit averaged a recovery rate of roughly 75% with less than 7% ash at a residence time of roughly 2 min., Eriez Flotation reported. The results led to the installation of a full circuit with rows of units.
The field testing came after Eriez was tapped to upgrade a customer’s flotation circuit. The coal particle size targeted was minus-150 microns, considered a coal slime.
Eriez first launched bench tests, which proved the potential viability of the solution. The solution averaged a 70% combustible recovery with less than 7% ash at a residence time of roughly 90 seconds.
Pilot-scale tests followed, validating the results. Three units in a series were trialed. Testing found that the circuit averaged combustible recovery in the range of 75% at less-than-7% product ash at less than 2 min. residence time, Eriez reported.
The results prompted the installation of two rows of three model SC-70 units, which are predicted to produce the same results.
Eriez said the StackCell is “the key technology” for treating often-rejected fraction, or for by-zero flotation without cyclone classification. “It is designed to handle exceedingly high volumetric flow rates and expedite the rate at which fine coal particles are floated,” said Drew Hobert, director of operations, flotation, Eriez. “The StackCell was created for by-zero coal flotation.”
The two-stage flotation solution “features a tank inside a tank with a one-way isolation of fluid between the tanks,” Hobert said.
“The new bubble-particle contact canister has high-energy dissipation for collection,” he said. “The external tank has low-energy quiescent conditions for froth recovery.”
The solution “increases air bubble concentration using maximum air flow and focused air introduction,” Hobert said. Since it “maximizes particle concentration, maximum hydrophobic species present,” he said. It “increases specific energy input by concentrating energy input, eliminating recirculating pumping, and using energy only for shearing and contacting.”
It offers “column-like performance, plug-flow behavior when in a series, and small cell volume,” Hobert said. It has a “low profile, but with high surface area, low energy demand, and low capital and operational costs.”
Compared to conventional flotation equipment used for by-zero feedstocks, “the smaller footprint, lower initial capital investment and projected water and energy savings make StackCell a smart business decision,” Hobert said. “StackCell offers several major advantages.”
At the top of the list is the “substantial reduction in total installed cost, including reduced building envelope as well as foundation loads and structural requirements,” he said. “Users also experience both reduced operational expenses and increased brownfield expansion opportunities.”
The solution features a smaller footprint. “Lift-out height is 42% less, diameter is 25% less, row length is 40% less, total envelope and footprint is 52% and 54% less,” Hobert said. “Train mass is 73% less,” he said. “Installed power is 38% less.”
Improving technologies and the potential for rising coal prices prompted the initial development of the solution more than 15 years ago.
“As a result of significant improvements in dewatering technologies, such as solid bowl centrifuges and filter presses, traditionally rejected slimes fractions that possess potentially high economic values,” Hobert said.
The StackCell was originally introduced in 2007. “Based on a complete understanding of flotation fundamentals and field experience, a new flotation cell was developed for fine and ultra-fine material to overcome the inherent limitations of existing technologies,” Hobert said.




